How to get started?
Even though effective and proactive terminology management comes with a great number of benefits in terms of improved quality, time and cost savings as well as global regulatory compliance, doing it right requires a collective and continuous effort. Terminology is a cross-functional issue and the costs of communication failures are often hidden and go un-owned, which is why it requires managerial and executive attention in order to undertake a more organized and focused approach.
To get started, you need to address three related areas:
Developing a company-specific business case (cost/benefit)
“Getting on your feet” and starting to make terminology management part of business-as-usual
Next, select a web-based terminology management system that is easy to implement and accessible from anywhere. As part of the regulatory requirements, the system must support detailed edit traceability, user management, version control, user discussions, and an automated workflow. In addition to these functions, you should also check the following:
No 3rd party software: having web access should be all that you need to harness the power of terminology management.
Scalability on-demand: adding users as you go, never overpay for something you don't need today.
No vendor lock-in: cancel anytime, don't get locked-in with complex software installations.
Remember that although the right tools are critical, success also requires the right people and processes, so you need to make sure important stake holders are aligned with your end goals. If needed, there are 3rd party consultants that are available to train your people on-site on the importance of company-wide terminology management and industry best practice.
The following diagram illustrates the process a terminology system, such as TermWiki Pro, can be integrated with a company’s existing content management workflow.
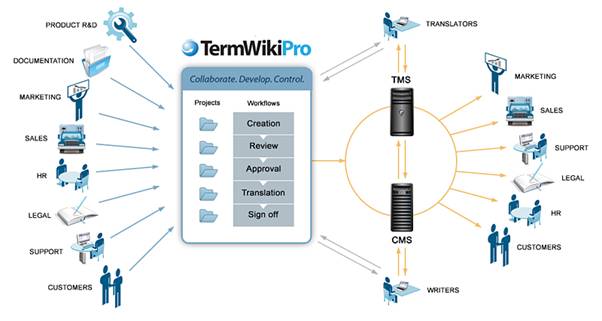
The end goal of implementing a terminology solution is to furnish a platform in which a company can both leverage existing multilingual content, and organize it within a framework that promotes the creation and ongoing collaborative development of new content. This will enable the company to achieve an optimal degree of consistency across documentation and languages, leading to considerable savings in both time and money, all the while ensuring compliance with global regulations.